The objective of the project is to develop segmentation techniques to estimate organ volumes that are of interest in both diagnostic and therapeutic nuclear medicine and to compare various techniques used in different hospitals to estimate thyroid volume. This parameter is of special interest in dosimetry calculations for patient-specific dose planning. The effect of different parameters such as fixed thresholding, automatic thresholding, attenuation, scatter, and reconstruction filter were also investigated.
When accurate quantitation is desired, detector response compensation and attenuation and scatter corrections become necessary. Obstacles to absolute quantitation with SPECT and recently developed methods attempting to overcome them have been investigated with special emphasis on attenuation and scatter correction techniques.
What are Monte Carlo Simulations ?
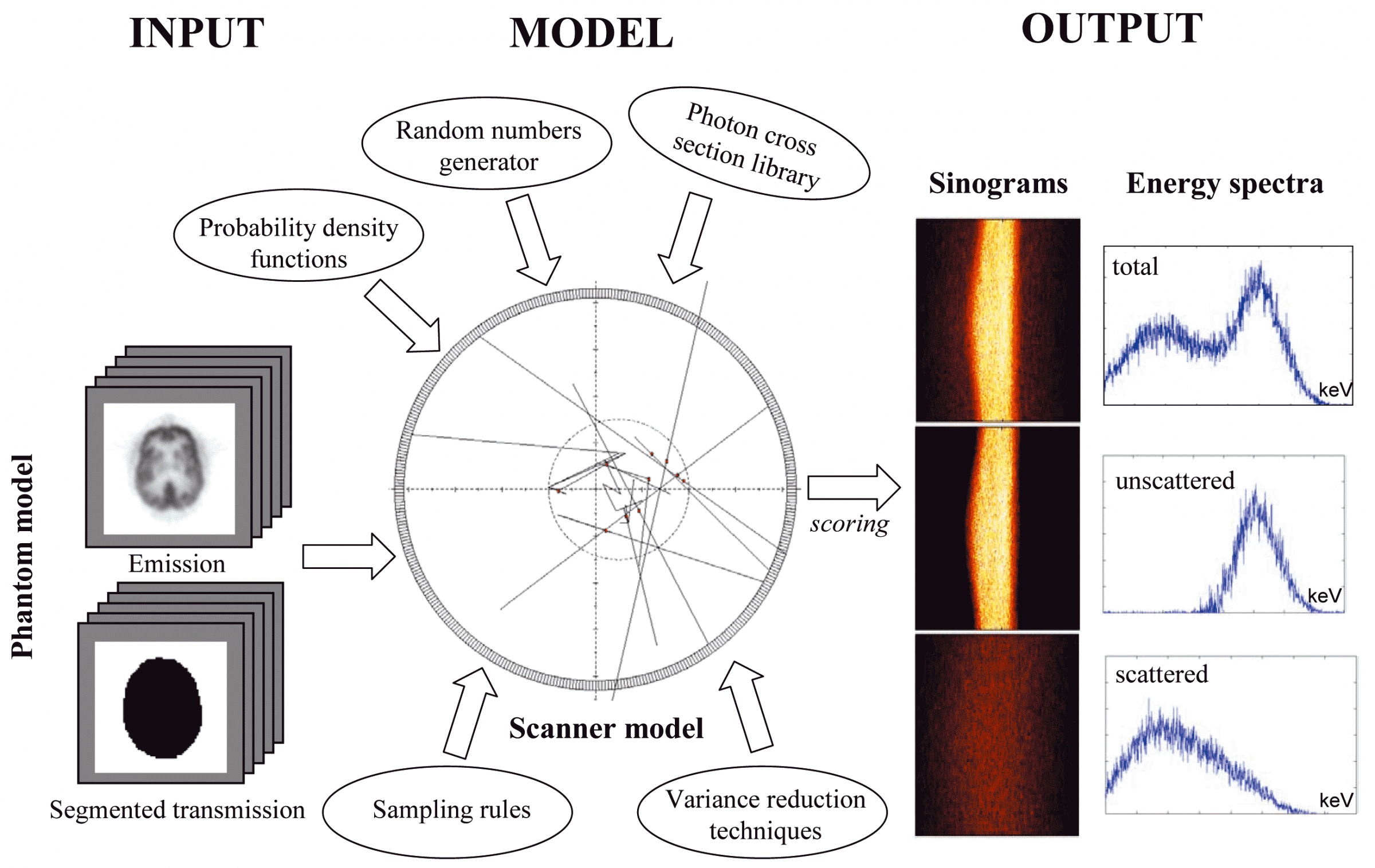
The objective of the project is to develop an accurate simulation tool of current generation 3D PET and CT scanners using sophisticated Monte Carlo techniques. Monte Carlo Simulations are used in a wide variety of fields. They are mathematical experiments in which statistical probabilities, random numbers, and computers are combined to solve problems that would otherwise be difficult, or even impossible to solve by other methods. Medical Physicists use the Monte Carlo method to simulate the random trajectories of radiation particles, such as photons, and electrons. By simulating a large number of these trajectories, detailed information can be obtained regarding the influence of new scintillating materials and other geometric configurations for PET design. It goes without saying that Monte Carlo studies are easier and cheaper than prototyping. Monte Carlo simulations have been shown to be very useful for evaluation of attenuation and scatter correction techniques since it is possible to obtain separate images of primary and scatter events. Also, a reference image without attenuation and scatter effects could be generated and used as a reference to which corrected images should be compared.
The original Monte Carlo simulator was written in Objective-C (Object-oriented programming language) and runs under NEXTSTEP 3.3. A phantom collection has been generated using simple source geometries and distributions as well as more clinically realistic source distributions. The model includes Compton scattering, attenuation, and non-ideal detectors as well as simulation of anthropomorphic voxel-based computer phantoms based on tissue composition and knowledge of the density distribution. The software package has been succesfully ported to other computer platforms and operating systems and parallelised on the Parsytec high performance parallel processing system. All that the user needs is the freely available GNU C compiler to use the Eidolon (nouveau site ??) package.
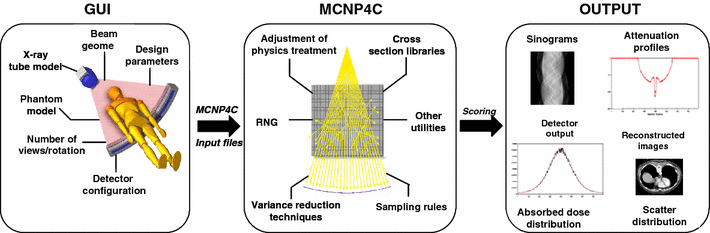
The CT Monte Carlo simulator is based on the general-purpose Monte Carlo N-Particle radiation transport computer code (MCNP4C). MCNP is a general-purpose Monte Carlo code that can be used for neutron, photon, and electron or coupled neutron/photon/electron transport. The main motivations behind the choice of this code are its wide use by the medical physics community, wide acceptance as an international standard for coupled particle transport having the best condensed history electron physics package, remarkable tally capabilities in addition to a powerful reporting system of statistical checks. Important features that make MCNP very versatile and easy to use include a powerful general source, critical and surface sources; both geometry and output tally plotters; a rich collection of variance reduction techniques; a flexible tally structure; and an extensive collection of cross section data. The latest version of the MCNP4C code released in 2000 incorporates many enhancements and new features such as EPE (electron physics enhancement) and the new el03 electron library for electron transport, which might influence the result of x-ray spectrum simulation. Electron physics enhancement was adopted to make MCNP more consistent with the Integrated Tiger Series (ITS) code. Monte Carlo simulation of x-ray spectra has been used extensively in different medical imaging applications including assessment of image quality, optimisation of system design and absorbed dose calculation. The accurate estimation of x-ray spectra is useful for x-ray tube design and correction of imaging artefacts such as beam hardening effect in CT. Our group developed a Monte Carlo simulation system for x-ray CT scanners. The validation phase focused on the simulation of x-ray spectra to be used as input to the CT simulator and its comparison to IPEM report 78 and comparison of simulated and experimental data generated for both fan-beam and cone-beam x-ray CT scanners. We now have a fully functionning PET/CT simulator that allows to assess typical artefacts and the quantitative imaging capability of dual-modality PET/CT imaging.
The objective of the PARAPET project is to make a significant contribution to medical imaging techniques by developing improved methods for volume reconstruction. This will involve the development of advanced new algorithms which can only be run cost-effectively on parallel systems. The project concentrated primarily on positron emission tomography (PET) which presents significant algorithmic challenges. The algorithms must run quickly in order to achieve satisfactory rates for those high-quality reconstructions necessary to improve clinical procedures and diagnostic techniques. Current and anticipated data rates and scanner resolutions and the new numerically intensive algorithms needed to produce high quality images preclude the use of current single-microprocessor systems and those likely to be available within the next five years. In order to make the proposed breakthrough in image reconstruction it was necessary to use high-performance parallel systems, an area in which Europe has a strong tradition and manufacturing base.
The project involved leading players from the UK, the Hammersmith Hospital, and Italy, the Ospedale San Rafaele, Milan, who are at the forefront of the development of clinical techniques related to PET. The coupling of this expertise with the strong algorithm capabilities of Brunel University, UK, and with the parallel processing expertise of Parsytec, Germany, the leading European supplier of embedded parallel processing hardware, will contribute to the development of an advanced European volume reconstruction capability. The project is strengthened through the collaboration of theGeneva University Hospital, Switzerland, who bring significant PET and parallel processing expertise into the project and who will contribute strongly to the end-user focus of the algorithmic development, and the Optimization Laboratory at the Faculty of Industrial Engineering and Management, Israel Institute of Technology, Haifa, Israel, who contribute to state-of-the-art expertise in reconstruction algorithms extending their work in this area already funded by industry. The project has a strong European dimension bringing together leading researchers, leading clinicians and a leading hardware vendor from across the EU in a collaboration strengthened by the participation of peer organisations in Switzerland and Israel. The image reconstruction library is now freely available as an open source software called STIR and is maintained by Kris Thielemans. See the credits page for a list of programmers who contributed to this project.
An article entitled: Parallel PET reconstruction methods give accurate diagnostic analysis describing the relevance of the project has been published in Virtual Medical Worlds.
The objective of the project is to develop new scatter correction techniques in 3D PET and to evaluate their performance as compared to more established correction methods using Monte Carlo simulated data, experimental phantom studies and clinical data. Accurate scatter correction is one of the major problems facing quantitative 3D PET. Increasingly sophisticated scatter correction procedures are under investigation, particularly those based on accurate scatter models, and iterative reconstruction-based scatter compensation approaches. The main difference difference the correction methods is the way in which the scatter component in the selected energy window is estimated. Monte Carlo methods give further insight and might in themselves offer a possible correction procedure.
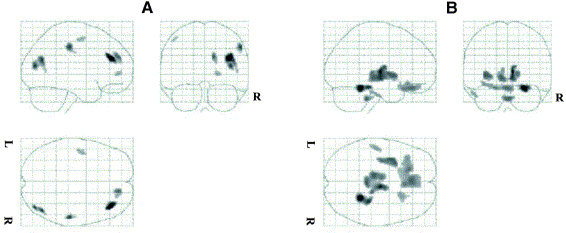
A new scatter correction method called Statistical Reconstruction-Based Scatter Correction (SRBSC) based on estimating the low-frequency component corresponding to scatter events using ordered subsets - expectation maximisation (OSEM) reconstructions was proposed and compared to the dual-energy window (DEW) technique, the convolution-subtraction (CVS) method, and two variants of the Monte Carlo-based scatter correction technique (MCBSC1 and MCBSC2). Among the conclusions drawn from this research is that all correction methods significantly improved the image quality and contrast compared to the case where no correction is applied. Generally, it was shown that the differences in the estimated scatter distributions did not have a significant impact on the final quantitative results. The DEW method showed the best compromise between ease of implementation and quantitative accuracy, but significantly deteriorates the signal-noise ratio. In addition, the effect of model-based scatter correction using the single-scatter simulation algorithm and iterative reconstruction in 3D brain PET studies were also assessed using statistical parametric mapping (SPM) analysis.
Statistical parametric maps resulting from the comparison of images reconstructed using model-based scatter correction and those corrected for attenuation using an effective linear attenuation coefficient without explicit scatter correction normalized using the [18F]-FDG template showing areas of significant regional decreases (A) and increases (B) in brain metabolism.
 The objective of the project is to develop improved attenuation and scatter correction techniques and their incorporation in iterative reconstruction for clinical whole-body 3D PET studies. The latest development in attenuation correction uses a segmented attenuation correction approach to reduce scan time and to improve final emission image quality. In this approach, a short transmission scan is acquired and the background corrected transmission image is reconstructed. Although the scan time and patient dose is reduced drastically, a short transmission scan typically suffers from considerable statistical noise because of low data counts. The filtered backprojection reconstruction technique may be too noisy for a successful segmentation.
The objective of the project is to develop improved attenuation and scatter correction techniques and their incorporation in iterative reconstruction for clinical whole-body 3D PET studies. The latest development in attenuation correction uses a segmented attenuation correction approach to reduce scan time and to improve final emission image quality. In this approach, a short transmission scan is acquired and the background corrected transmission image is reconstructed. Although the scan time and patient dose is reduced drastically, a short transmission scan typically suffers from considerable statistical noise because of low data counts. The filtered backprojection reconstruction technique may be too noisy for a successful segmentation.
We developed a powerful and robust segmentation tool based on the fuzzy-clustering approach, the use of which in combination with morphological information provided by the cluster (sharp boundaries) to improve homogeneity within regions allows to reduce significantly the noise. Short transmission images in whole-body scanning can therefore be segmented into regions (clusters) of known attenuation coefficients (e.g. lungs, soft tissue and air). Fuzzy clustering-based segmented attenuation correction in PET shows a clear reduction of noise propagation from transmission into emission data, allowing for reduction of transmission scan duration. More recently, we developed a new method for determination of the attenuation map in brain PET using coregistered MRI and made an exhaustive comparative evaluation of the effect of the attenuation map on absolute and relative quantification in functional brain PET imaging using clinical data. Although the impact of accurate physical correction techniques on accurate quantification of brain function is still controversial, the extent of this effect is, however, a challenging issue that certainly requires further research and development efforts.
Statistical parametric mapping (SPM) is now considered the gold standard both in research and clinical neuroimaging investigations. However, there are few simulation studies in both PET and SPECT and a single experimental study that were carried out to validate the methodology. This project aims at investigating activation focus characteristics (size, activation/lesion intensity and location), and influence of different data correction (attenuation and scatter, partial volume effects), study size (number of subjects, number of controls), and reconstruction (analytic, iterative) techniques on the sensitivity and specificity of an SPM analysis in PET neuroactivation studies. Both Monte Carlo simulations and experimental measurements are being used.
We have constructed a set of spherical activation foci of different size using a recently developed technique that can be used to construct wall-less small radioactive lesions using moulted wax (1). These spherical activations ranging from 4 to 12 mm (in steps of 2 mm) and with 18F activity ranging from 0 to 80% will be inserted in the gyrus cingulate overlapping both hemispheres, right frontal cortex, right supramarginal gyrus, left putamen, and left thalamus within the digital and physical Hoffman 3D brain phantoms.
A more recent study aimed at assessing the effect of model-based scatter correction (SC) in 3D brain PET studies using SPM analysis in healthy volunteers. The images were coregistered and normalized using the default 15O-[H2O] template supplied with SPM99 and an 18F-[FDG] template. A t statistic image for the contrast condition effect was then constructed. It was concluded that significant differences in 18F-[FDG] distribution exist when images are reconstructed with and without explicit SC for some cerebral areas. This needs to be acknowledged for adequate interpretation of 18F-[FDG] 3D brain PET images after applying SC.
Quantification of brain function using PET is an emerging research field. For many years, interest in the regional distribution of brain perfusion and metabolism has steadily increased, primarily because many neurological disorders are associated with a decrease of regional cerebral blood flow. PET offers the possibility of quantitative measurements of tracer concentration in vivo. However, there are several issues that must be considered in order to fully realize this potential. In practice, the measured line integrals must be corrected for a number of background and physical effects, the three most significant being the limited spatial resolution and associated partial volume effect, photon attenuation, and the contribution of events arising from photons scattered in the patient and gantry.
The aim of this project is to develop a strategy for the improvement of three-dimensional (3D) brain positron emission tomography (PET) quantification of brain function using sophisticated image analysis and processing tools combining both functional (PET) and anatomical (MRI) imaging with potential applications in the study of Parkinson neurodegenerative disease.
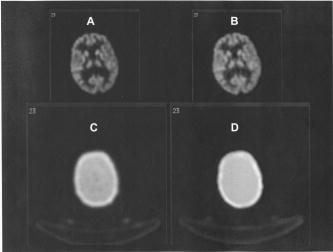
Comparison of attenuation maps and PET image slices of a patient study reconstructed using the two different processing protocols. (A) PET image reconstructed using transmission-guided attenuation and scatter corrections. (B) PET image reconstructed using segmented MRI-guided attenuation and scatter corrections. (C) Measured transmission-based attenuation map. (D) MRI-guided attenuation map.
To improve the capability for investigating the living human brain using PET with regard to blood flow, metabolism and receptor characteristics for small structures, the spatial resolution has to be improved relative to what is available today. A spatial resolution of 2 mm or less in all three dimensions over the entire field of view (FOV) and recording the depth of interaction (DOI) information may be necessary to reach these goals. To meet this research challenges, new next generation high-resolution 3D-only brain tomographs have been designed or are under development by different research groups.
The CIMA collaboration (Computer Imaging for Medical Applications) is actively engaged in a novel 3D PET design concept, which is expected to provide higher performance at comparable or lower cost than existing operational scanners or known prototypes under development elsewhere.The proposed concept is made possible by the latest developments of advanced instrumentation carried out at CERN for the future Large Hadron Collider (LHC). The use of large area and highly pixelized Hybrid Photon Detectors (HPDs) with enclosed VLSI self-triggering electronics allows for a cost efficient readout of finely segmented arrays of scintillator crystals. We are collaborating with CERN, which is equipped with facilities to develop and build high-performance HPDs optimised for PET applications. The concept is based on a PET detector module consisting of a stack of relatively long scintillation crystals read out on both sides by proximity focused HPDs. A promising application of the proposed camera module, which is currently under study is a high resolution brain PET with an axial field of view of 10-15 cm dedicated to brain research. Other applications like PE mammography (PEM) and combination with other modalities are also under investigation.
Illustration of a brain PET scanner based on 12 camera modules.
Dual-modality PET/CT imaging is an approach that combines imaging modalities in a way that provides diagnostic information that is not available from a single imaging modality alone. A significant success of PET/CT has been the improved image quality of FDG images for tumour localization. Dual-modality imaging also has important ramifications for radiotracer quantification and image-guided radiation therapy treatment planning, the major focus of this project.
This project aims to develop strategies for improvement of tumour detectability in clinical oncology using dynamic positron emission tomography (PET) acquisition protocols combined with multivariate image analysis and nonlinear similarity measures, and to implement the techniques and methodology required for accurate and reproducible dual-modality PET/CT-guided radiation therapy treatment planning incorporating automated image segmentation techniques for target volume delineation and robust partial volume correction techniques for accurate lesion quantification as well as protocols designed to reduce respiratory motion artefacts in PET images of the upper abdomen and lung cancer thus enabling correct localization and accurate quantitation of lesion size and radiation dose delivery to the target volume.
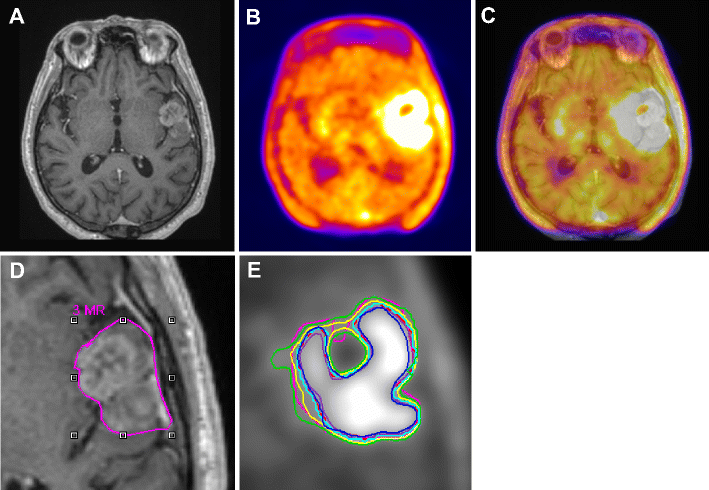
(A) Gadolinium enhanced T1-weighted MRI
(B) corresponding 18F-FET PET, and fused PET/MR
(C) transaxial slices of a clinical study with a glioblastoma showing differences in target-volume definition.
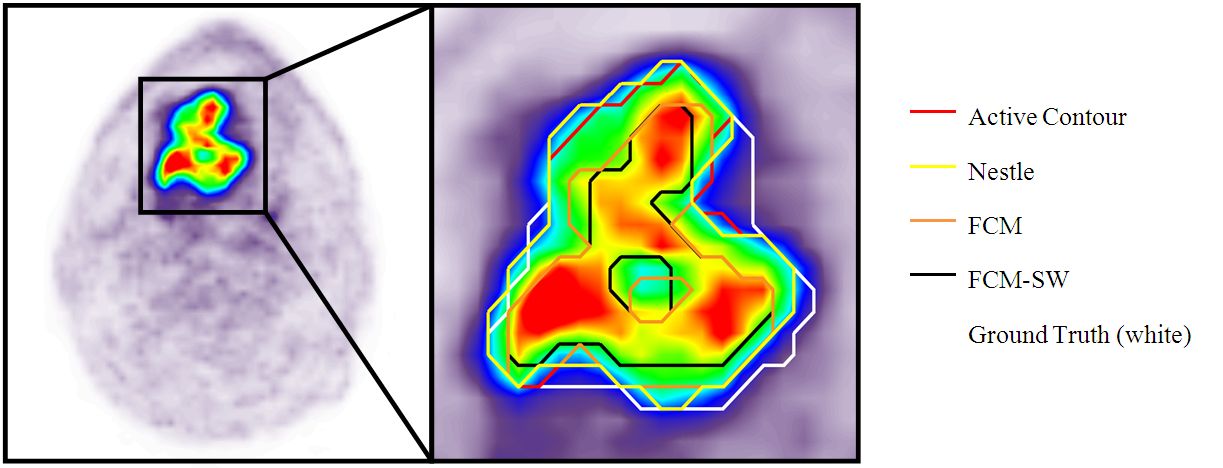
Indicated are (D) the gross tumor volume (GTV) delineated on MRI (GTVMRI), and (E) enhanced details of PET-based GTVs obtained by manual delineation of contours (GTVman; magenta), an isocontour of a standardized uptake value (SUV) of 2.5 (GTV2.5; purple), a fixed threshold of 40% (GTV40%; green) and 50% (GTV50%; cyan) of the maximum signal intensity, signal-to-background ratio (SBR)-based adaptive thresholding (GTVSBR; yellow), gradient find (GTVGF; blue), and region growing (GTVRG; red) segmentation algorithms.
Note that GTVMRI overestimates the tumor extension relative to GTVman.
PET images generated by the preclinical dual-modality PET/CT system (Gamma Medica-Ideas) installed in our department - funded by the “Centre d’Imagerie Biomédicale” (CIBM) - suffer from low spatial resolution characteristics and are nonquantitative owing to the lack of sophisticated image correction and reconstruction techniques on software supplied by the scanner manufacturer. Multimodality molecular imaging has become an essential tool for the development of new tracers, to study the molecular pathways of disease, including factors such as gene expression, in living subjects, and to test new therapeutic approaches in animal models of human disease. Multimodality imaging also has important ramifications for quantification of molecular targets of disease states, which is the major focus of this project. For example, dual-modality PET/CT imaging provides x-ray CT data which can be normalized to obtain a patient-specific map of attenuation coefficients that can be used to compensate the correlated radiotracer data for photon attenuation or for Compton scatter. In addition, regions of interest defined anatomically on the CT image can be used to quantify the correlated radiotracer data in a way that allows more precise target and background definition, and that can use model-based methods that correct the extracted quantitative data for partial-volume effects and other physical perturbations. However, there are several challenges that still face the use of preclinical PET/CT imaging, and that may represent inherent limitations in this technique and remain hot topics undoubtedly still requiring further research and development efforts, some of them will be addressed in this research project.
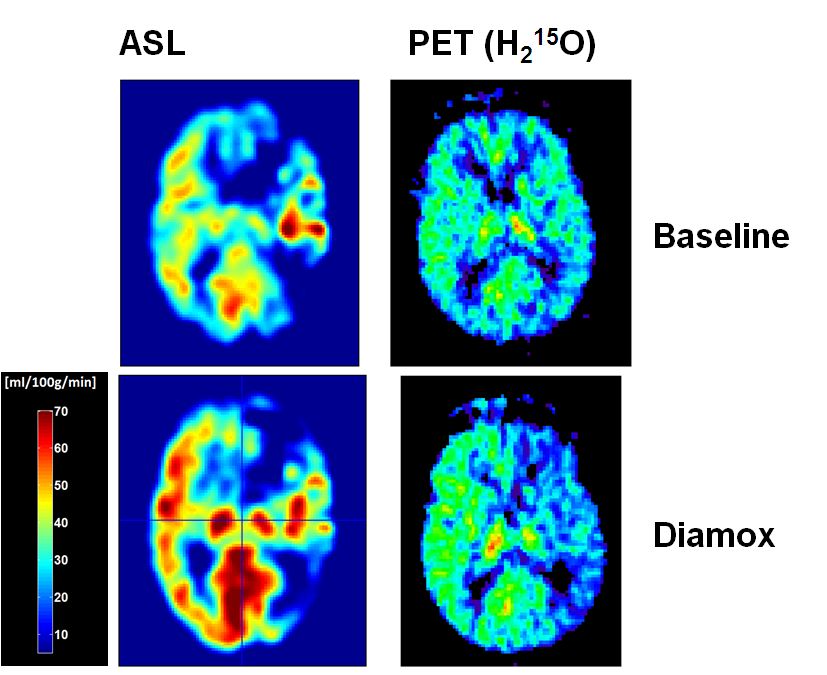
This project is intended to investigate the hemodynamic effects and outcome in carotid artery disease (CAD) by measuring the cerebrovascular reserve capacity (CVR) in the whole brain by means of perfusion imaging. Arterial spin labelling (ASL) perfusion imaging offers the advantage to measure global and regional perfusion changes induced by CAD non-invasively and repetitively without the need to apply a tracer substance. Positron emission tomography (PET) using H2O-15, is the gold standard of brain perfusion measurements but not routinely available in clinical practise, therefore not suited for repetitive examinations. Implementation of ASL in the measurements of CVR may provide non-invasively a high resolution whole brain map of CVR and therefore qualify as routine measurement in the diagnostic workup of CAD3. As there is still a lack in reliable data, regarding I) the validation of ASL-CBF measures in patients with CAD4 and II) the risk and benefits of CAD and its revascularisation, this consortium of neurology, neuroradiology, vascular surgery and nuclear medicine experts will approach the yet unresolved questions of providing clinical reliable CBF measures in elderly patients with CAD by means of ASL and to investigate CAD-related neuropsychological impairment and benefits of revascularisation.
The research project comprises two subprojects that are intimately connected. In both of them different research groups will cooperate on the same topic and combine specific competences to optimize the benefit of the study as a translational cooperation.
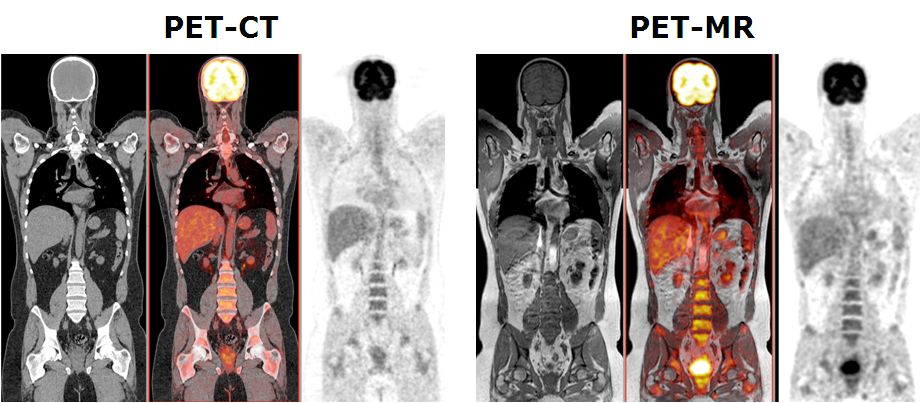
This project aims to develop quantitative procedures for dual-modality PET-MR imaging. As diagnostic techniques transition from the systems to the molecular level, the role of multi-modal imaging becomes increasingly important. The combination of positron emission tomography (PET) and magnetic resonance imaging (MRI), enabling truly simultaneous acquisition, is expected to bridge the gap between molecular and systems diagnosis. MRI and PET offer richly complementary functionality and sensitivity; fusion into a combined system offering simultaneous acquisition will capitalise the strengths of each, providing a hybrid technology that is greatly superior to the sum of its parts. A prototype clinical whole-body dual-modality PET-MR system is installed in our department since Feb. 2010 by one of the leading commercial players in molecular imaging instrumentation (Philips Medical Systems, OH, USA) for characterisation, validation, refinement and collection of PET-MR data for clinical research. This project aims to the improvement of the adopted sequential design of the hybrid PET-MR technology towards a fully integrated PET-MRI hybrid system allowing simultaneous collection of PET and MRI data as well as to develop the software tools required for accurate fusion and quantitative analysis of clinical data PET-MR sets. This work will push forward the development and use of PET-MRI - an embryonic technology that has excellent potential to become a powerful tool for preclinical research and clinical diagnosis.
The aim of this project is to develop strategies for multitracer molecular imaging of tumour metabolism, cell proliferation and hypoxia and for the improvement of the process of PET/CT-guided radiation therapy treatment planning incorporating automated image segmentation techniques for target volume delineation and identification of hypoxic regions. While most cancer therapies deliver a uniform amount of radiation to the tumour as a whole, malignant lesions are not homogenous. It is hypothesized that most of tumours contain three statistically different subpopulations with distinct profiles. We plan to evaluate multitracer PET/CT scans of series of patients and measure three different characteristics, namely: metabolism, cell proliferation and oxygen deprivation of hypoxia. These three factors can vary within a tumour to effect how a tumour reacts to treatment. Three different PET tracers as surrogates for the different characteristics will be used, namely: 18F-FDG for metabolism, 18F-FLT for cell proliferation and 18F-FAZA/18F-FMISO for hypoxia. Computational algorithms will be developed to classify the regions based on these three parameters.
Current procedures for acquiring clinical PET-CT data in many departments for the paediatric population are still based on similar protocols than for adults, thereby increasing radiation dose and observer variance. Dose reduction techniques combined with methodological developments aiming at improving image quality and automatic quantitative assessment of functional data is attractive and will certainly revolutionize our practice of functional oncological imaging since it can lower variability across institutions and may enhance the consistency of image interpretation independent of reader experience. The main aim of the present project is to develop the techniques and strategies required for reducing the absorbed dose without hindering image quality and quantitative accuracy in paediatric dual-modality PET/CT scanning. This can be achieved through the development of efficient scanning protocols adapted to the paediatric population.
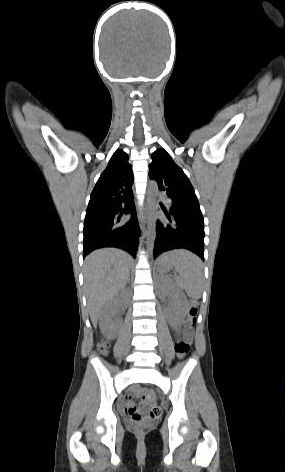
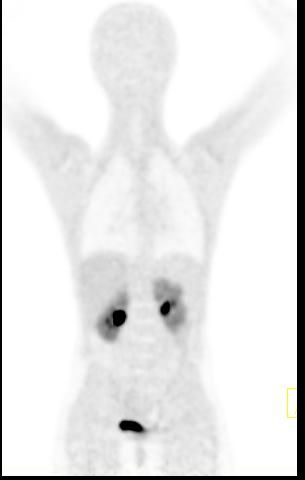
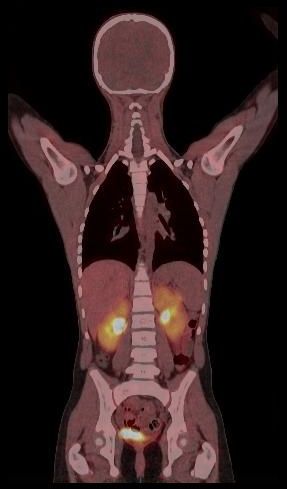
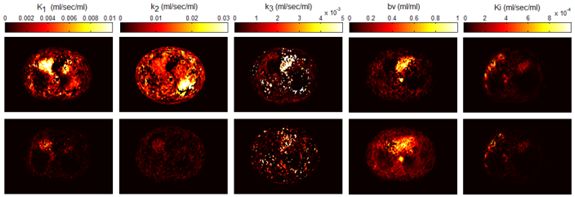
Whole body hybrid (PET-CT and PET-MR) imaging, making use of the standardized uptake value (SUV) index, are well established in clinical setting for diagnosis and staging, treatment response monitoring and radiation therapy treatment planning of a wide range of oncologic malignancies. However, the SUV metric derived from static PET data does not capture the dynamics of the PET probe biodistribution in the body. Alternatively, dynamic PET imaging, presently employed mostly in research setting, is capable of tracing tracer kinetics thus allowing for more accurate quantification of tracer uptake. This acquisition mode enables to enhance, in certain situations, normal and abnormal (tumour) tissue characterization, assessment of treatment response or radiation therapy planning.