On 14 August 2024, the World Health Organization (WHO) declared the current situation regarding the mpox virus to be a Public Health Emergency of International Concern (PHEIC). (The original declaration can be consulted at the following address: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern).
Mpox is not a new virus. It has been known to infect humans since the 1970s. It was previously referred to as monkeypox, because monkeys were accidentally infected, and the name was recently changed to ‘mpox’. The natural reservoir of the virus is not monkeys, but rodents endemic to West, Central and East Africa. In these regions, there has been an increase in cases of mpox since vaccination against human smallpox ceased in the 1980s, a trend that has become even more marked since the global epidemic of 2022.
It was the sharp increase in the number of cases reported in the Democratic Republic of Congo (DRC) and a growing number of previously unaffected neighbouring countries, and the emergence of a new clade of the virus (a new strain that has evolved), called Clade Ib, that prompted the PHEIC declaration. Current challenges in the region include a lack of access to diagnostic tests, vaccines and treatments. The WHO and other partners are working with countries and manufacturers to address this need and bring the epidemic under control.
Currently, several epidemics caused by different strains are underway simultaneously in many countries, with distinct modes of transmission and levels of risk. There are still uncertainties regarding the case-fatality rate and morbidity, as well as the transmissibility of the different strains of virus, given the paucity of existing data on mpox and on the current epidemic in East Africa.
The overall risk to the general public is currently considered low by the ECDC for the EU/EEA (https://www.ecdc.europa.eu/en/publications-data/risk-assessment-mpox-epidemic-monkeypox-virus-clade-i-africa). It is likely that cases will be diagnosed in Switzerland and Europe in the coming weeks.
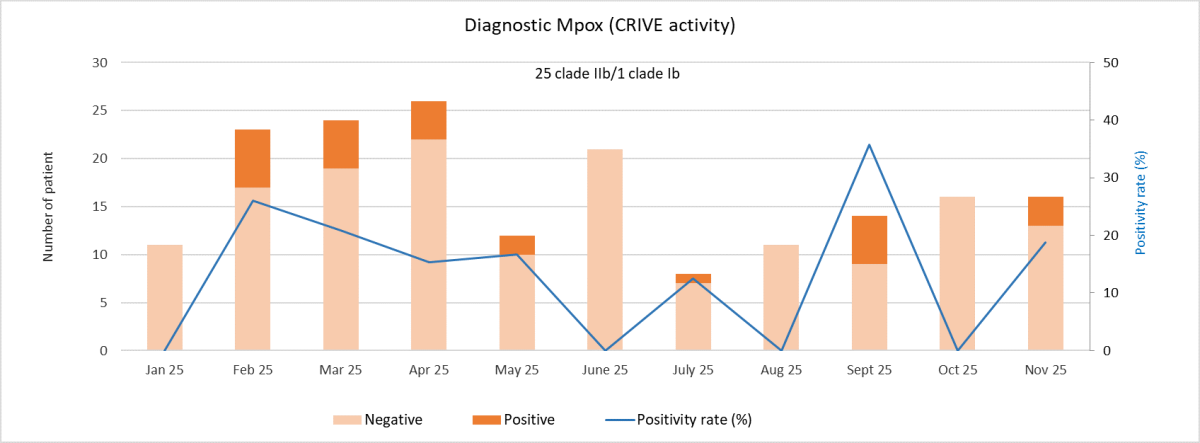
Our Center is actively engaged in the diagnostic validation of mpox tests with our partner FIND and as part of our activities as a WHO collaborating center.
Our reference laboratory, CRIVE, is able to test samples in case of clinical suspicion.
Additionally, clinical studies on mpox are currently underway at HUG.
Below is a brief summary of the epidemiological situation, as well as the case definition and contact persons at HUG in case of suspicion. Given the clinical presentation, the majority of patients consult as outpatients. More detailed information on the disease, diagnosis (sample collection) is available below.